Riboflavin Sodium Phosphate
5100.0 INR/Kilograms
Product Details:
- Heavy Metal (%) NMT10ppm
- Taste Odorless
- Boiling point 100 C
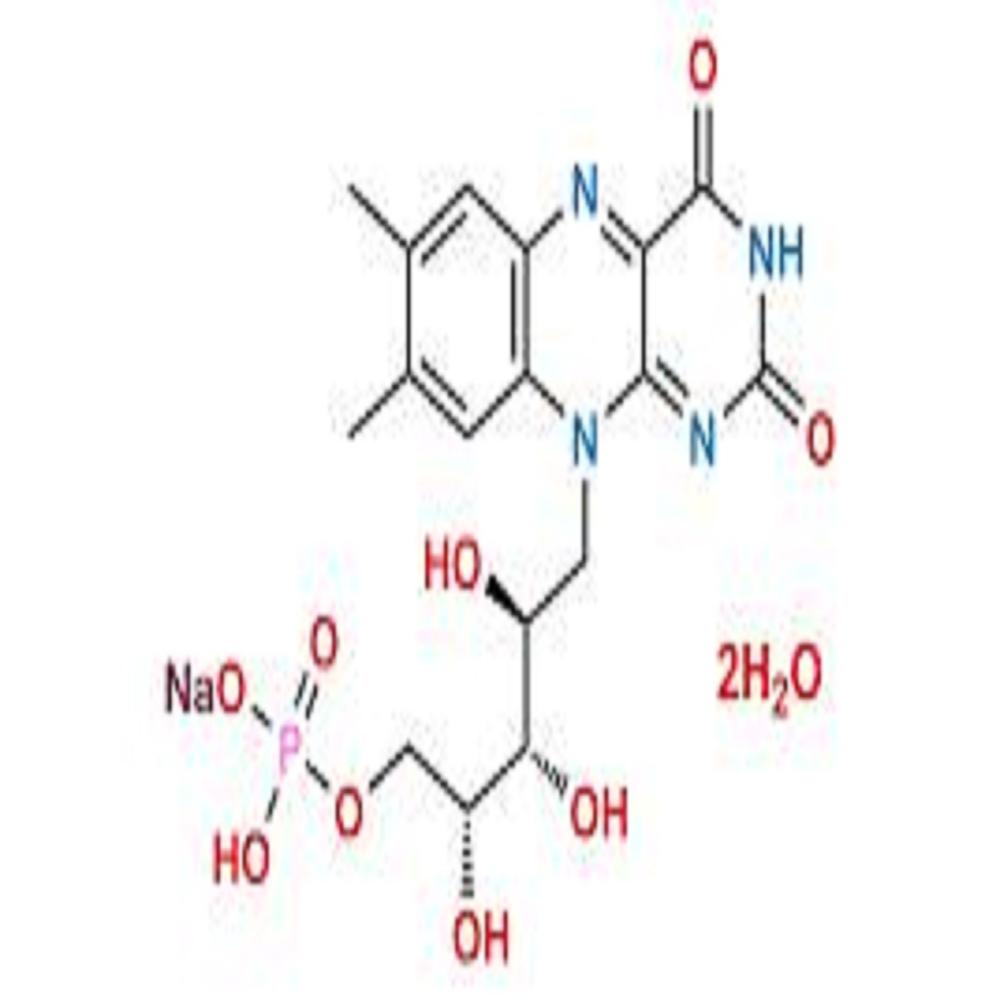
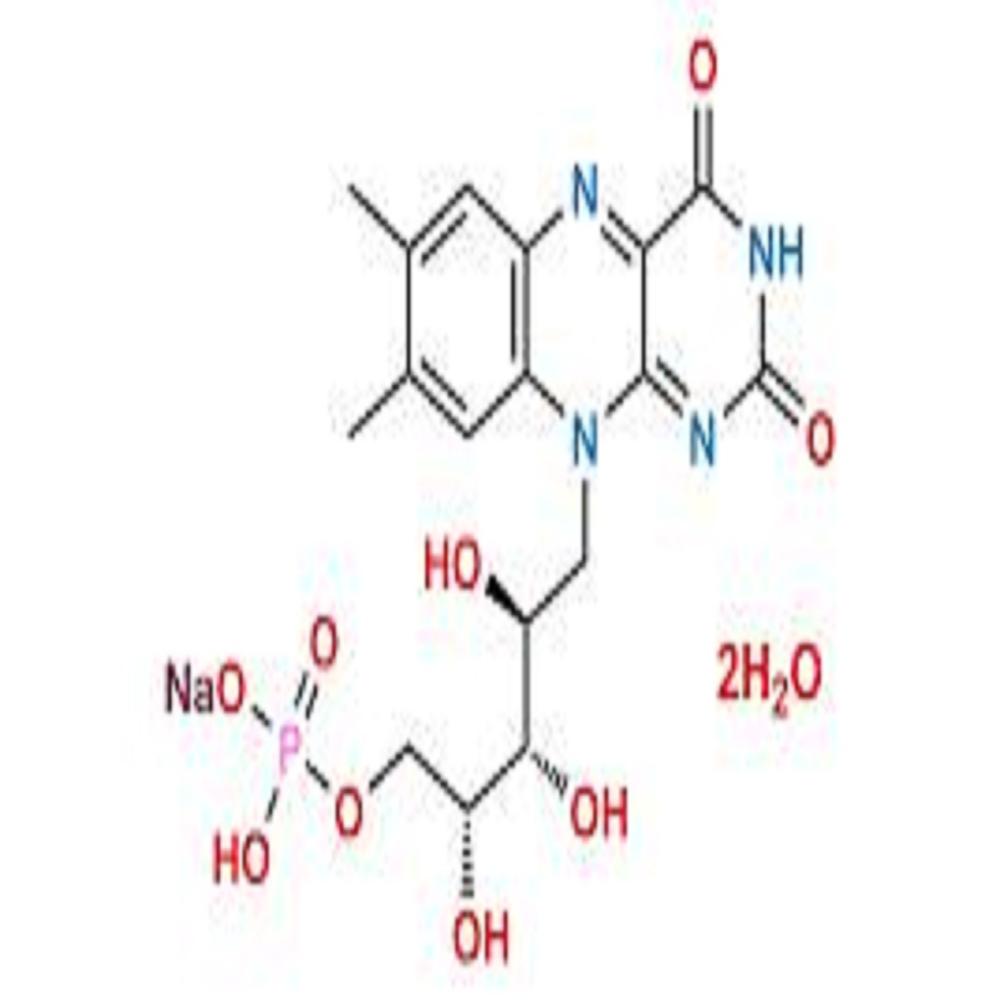
- Structural Formula C17H20N4NaO9P
- Molecular Formula C17H20N4NaO9P
- EINECS No 244-868-0
- Smell Other
- Click to View more
X
Riboflavin Sodium Phosphate Price And Quantity
- 5100.0 INR/Kilograms
- 25 Kilograms
Riboflavin Sodium Phosphate Product Specifications
- Odorless
- Riboflavin Sodium Phosphate is primarily used to treat or prevent riboflavin (vitamin B2) deficiency, which can lead to various health issues. It can be administered in different forms, such as oral supplements or injections, depending on the severity of the deficiency. Common uses include: Treatment of Riboflavin Deficiency: Riboflavin is essential for the body s metabolism, supporting healthy growth, vision, skin, and the functioning of the nervous system. Deficiency can lead to symptoms like sore throat, cracked lips, swollen tongue, and dermatitis. Supplementation for Specific Conditions: Migraine prevention: Some studies suggest riboflavin supplementation may help reduce the frequency of migraines. Eye health: Riboflavin plays a role in maintaining normal vision and is sometimes used to treat eye-related issues, such as cataracts. Injection Use: In cases of severe deficiency, riboflavin sodium phosphate may be administered via intravenous (IV) or intramuscular (IM) injections, especially if oral administration is not feasible due to conditions like malabsorption or in patients with difficulty absorbing oral medications. Prevention of Deficiency in High-Risk Populations: It is sometimes given to individuals who have a higher risk of riboflavin deficiency, such as those with chronic alcoholism, poor diets, or certain malabsorption disorders.
- NMT10ppm
- C17H20N4NaO9P
- 100 C
- Medicine Grade
- Riboflavin-5-Phosphate
- Powder
- 83-88-5
- C17H20N4NaO9P
- clear and yellow to orange-yellow in color
- NMT7.5%
- Sparingly soluble in water
- Other
- 5 Years
- 244-868-0
- 478.33 Kilograms (kg)
- 29362310
- (6,7-dimethyl-9-(D- -erythro-2,3,4-trihydroxybutyl)isoalloxazine) sodium phosphate.
- 99
- Pharmaceutical Intermediates
- Room Temperature
- 280 degree
- 5.0 and 6.5
Riboflavin Sodium Phosphate Trade Information
- india
- Days after Acceptance (DA), Letter of Credit at Sight (Sight L/C), Cash Advance (CA), Cash in Advance (CID), Letter of Credit (L/C)
- 100 Kilograms Per Day
- 7 Days
- No
- Free samples are available
- 25Kgs Drum/bag packing
- Asia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East
- All India
- WE PROVIDES ALL KIND OF CERTIFICATIONS AS YOU REQUIRED
Product Description
Riboflavin Sodium Phosphate is a water-soluble form of riboflavin (Vitamin B2) where riboflavin is combined with phosphate and sodium. This compound is often used in various pharmaceutical, nutritional, and food applications. It serves as a stable form of riboflavin, ensuring its bioavailability and effectiveness in the body.
Key Characteristics:
-
Chemical Composition:
- Riboflavin (Vitamin B2) is the core molecule.
- Sodium and phosphate are added to enhance stability and solubility.
-
Physical Properties:
- Typically appears as a yellow crystalline powder.
- It is water-soluble, making it easier to incorporate into liquids like supplements, intravenous (IV) solutions, and fortified foods.
-
Uses:
- In Pharmaceuticals: It is often included in IV fluids or injections for patients who require riboflavin supplementation (such as those with riboflavin deficiency or certain metabolic disorders).
- Nutritional Supplements: Added to multivitamin formulations or standalone riboflavin supplements to treat or prevent vitamin B2 deficiency.
- Food Fortification: Commonly added to cereals, dairy products, and beverages to enrich them with vitamin B2.
- Cosmetics: Sometimes used in skincare products for its purported antioxidant and skin-health benefits.
-
Benefits of Riboflavin:
- Essential for energy production (helping to convert carbohydrates into energy).
- Vital for the maintenance of healthy skin, eyes, and nervous system.
- Works as an antioxidant, helping to protect cells from oxidative damage.
-
Stability:
- The sodium phosphate component enhances the stability of riboflavin in solution, preventing it from degradation due to light and air exposure.
Typical Applications:
- Medical treatments for conditions like migraines (where riboflavin supplements are commonly prescribed).
- Used in intravenous infusions when there's a need for direct vitamin administration.
- In food industry, as a fortifying agent to boost nutritional content.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email







 : nilesh.sheth70
: nilesh.sheth70
