Loteprednol Etabonate
Product Details:
- Molecular Weight 466.9 Grams (g)
- Boiling point SLIGHT HIGHER THAN MELTING POINT
- Loss on Drying less than 1.0%
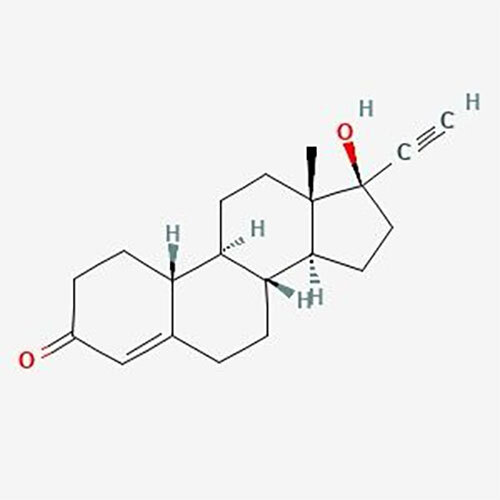
- Structural Formula C24H31ClO7
- Shelf Life 3 Years
- Solubility relatively limited in water but is soluble in organic solvents
- Melting Point 222 C to 226 C
- Click to View more
Loteprednol Etabonate Price And Quantity
- 25 Kilograms
- 150000.0 INR/Kilograms
Loteprednol Etabonate Product Specifications
- SLIGHT HIGHER THAN MELTING POINT
- Loteprednol Etabonate is a corticosteroid used primarily in the treatment of eye conditions. It is commonly prescribed to reduce inflammation and swelling in the eyes caused by surgery, injury, or other conditions like allergic reactions or eye diseases. It is often available in the form of eye drops. Here's a breakdown of its use: 1. Mechanism of Action: Loteprednol Etabonate works by inhibiting the action of substances in the body that trigger inflammation. As a corticosteroid, it helps decrease the production of inflammatory mediators and reduces swelling, redness, and pain. 2. Indications: Treatment of inflammation associated with ocular surgery. Managing allergic conjunctivitis. Treating inflammatory eye diseases such as uveitis. 3. Forms and Dosage: Eye Drops: Typically, it is applied 1 to 2 drops in the affected eye(s) 2 to 4 times a day, depending on the severity of the condition and the healthcare provider s instructions. Ointments or other forms might also be used depending on the specific condition. 4. Side Effects: Like most medications, it can cause side effects. Some potential side effects include: Eye irritation or discomfort Blurred vision Increased intraocular pressure (especially if used for extended periods) Potential for cataract formation with long-term use 5. Precautions: Glaucoma: Careful monitoring is needed for patients with glaucoma since corticosteroids can increase intraocular pressure. Infections: It may mask the signs of eye infections or exacerbate them. Pregnancy and Lactation: Always consult with a healthcare provider if you're pregnant or breastfeeding before using this medication.
- white or off-white cream or gel
- 466.9 Grams (g)
- less than 1.0%
- C24H31ClO7
- 29372900
- Solid
- 222 C to 226 C
- 120114-12-1
- Loteprednol Etabonate
- 3 Years
- relatively limited in water but is soluble in organic solvents
- Medicine Grade
- Bitter
- 98%
- Room Temperature
- not more than 10 ppm
- 539-586-7
- 5.0 to 6.0
- No Smell
- 1-10 microns
- C24H31ClO7
- Lotemax
Loteprednol Etabonate Trade Information
- mumbai
- Cash Advance (CA), Cash in Advance (CID), Letter of Credit at Sight (Sight L/C), Letter of Credit (L/C)
- 1000 Kilograms Per Day
- 7 Days
- No
- Free samples are available
- DRUM / BAG PACKING
- Asia, North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Australia, Central America
- WE PROVIDES ALL KIND OF CERTIFICATIONS AS YOU REQUIRED
Product Description
Loteprednol Etabonate manufactured by Shreeji Pharma International is supplied and exported from Gujarat unit to all over the world. The pure and effective product produced at the GMP site in line with the regulatory laws.
Technical Specifications:
- Superlist Name: Loteprednol etabonate
- Formula: C24H31ClO7
- Density: 1.31 g/cm3
- Melting Point: 220.5-223.5 C
- Molecular Weight: 466.96
- Synonyms: Alrex;CDDD 5604;HGP 1;Lotemax;
- Boiling Point: 600.1 C at 760 mmHg
- Flash Point: 316.7 C
Introduction of product:
Loteprednol etabonate (LE) is a corticosteroid developed according to Bodor's "soft drug" concept. Inflammatory ophthalmic conditions can be treated effectively with LE. LE is administered in sterile eye drop form. It is either available in 0.5% or 0.2% suspension.
How does it work:
Treatment of some eye conditions resulting from inflammation or injury is provided with this medication. In addition, it is used following eye surgery. Symptoms such as swelling, redness, and itching are relieved by loteprednol. The medication belongs to the group of drugs known as corticosteroids.
Applications Or where it is used:
Medications such as this one is used to treat certain eye conditions caused by inflammation or injury. This product is also used after surgery on the eye. In order to relieve swelling, itching, and redness, loteprednol relieves these symptoms. It belongs to the corticosteroid class of drugs.
How to use:
Put your head back, look up, and pull down your lower eyelid to form a pouch. Put 1 drop into the pouch with the dropper held directly over your eye. Spend a minute or two looking downwards and closing your eyes gently. Put your finger at the corner of your eye (near your nose) and gently press it.
Dosage of usage:
Please read the medication guide or instruction sheet that is on the back of your prescription label. Be sure to follow the instructions on the label exactly.
If you wear soft contact lenses, do not use this product. As a result of the preservative in loteprednol, the lenses could become permanently stained. Contact lenses should be inserted 15 minutes after using the medication.
Use this eye medication only after washing your hands.
Every time you use eye drops, shake the bottle well before using it. Shake the bottle once to fill the dropper tip with gel when using the gel.
Side effects:
When you have these symptoms of an allergic reaction, receive immediate medical attention. Hives, difficulty to breathe, swelling of face, lips, tongue, or throat are all signs of an allergic reaction.
Call your doctor at once in case of following:
- Eye drops cause eye pain
- Continuous redness or itching
- Eye pain or swelling, trouble in closing eye
- Pain behind your eyes or sudden vision changes
- Tunnel vision, seeing halos around lights
- Signs of eye infection discomfort, redness, crusting or drainage
Common side effects are:
- Minor burning on using eye drops
- Eye pain or blurred vision
- Sensitivity to light
- Headache
- Dry or watery eyes
- Feeling like something is in eye
Warnings and precautions while using this product
This medicine should not be used if you have an eye infection like herpes simplex.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+









 : nilesh.sheth70
: nilesh.sheth70
