Sodium Saccharin
Product Details:
- Storage Room Temperature
- Smell Other
- Molecular Formula C7H4NNaO3S
- Taste Sweet
- Boiling point 438.9 C
- Particle Size 3 micron
- Solubility saccharin is not water-soluble
- Click to View more
Sodium Saccharin Price And Quantity
- 25 Kilograms
- 600.0 INR/Kilograms
Sodium Saccharin Product Specifications
- 205.17 Grams (g)
- Pharmaceutical Intermediates
- Not Available
- 6155-57-3
- 231
- 2-7
- 99 percent
- C7H4NNaO3S
- 2925
- 3 Years
- Sodium Saccharin
- Saccharin is an artificial, or nonnutritive, sweetener that is used in the production of various foods and pharmaceutical products including: Baked goods. Jams. Chewing gum. Drinks.
- >300 C
- 204-886-1
- saccharin is not water-soluble
- 3 micron
- 438.9 C
- Sweet
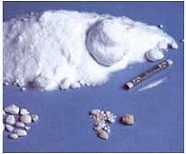
- Powder
- C7H4NNaO3S
- Medicine Grade
- Other
- Room Temperature
- C7H5NO3S
- white crystals or crystalline powder
Sodium Saccharin Trade Information
- Mumbai port
- Cash Advance (CA), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Letter of Credit (L/C)
- 50 Kilograms Per Day
- 7 Days
- No
- Free samples are available
- drum pack
- South America, Eastern Europe, Asia, Australia, North America, Western Europe, Middle East, Africa, Central America
- WE PROVIDES ALL KIND OF CERTIFICATIONS AS YOU REQUIRED
Product Description
Sodium Saccharin Powder
We are offering high quality of Sodium Saccharin Powder. We are known for manufacturing, exporting, distributing, trading and supplying Sodium Saccharin Powder in Gujarat, India. Further, this is safely packaged by our professionals in diverse packaging options that maintain its purity and effectiveness.
Shreeji Pharma International is leading Manufacturer, Exporter, Distributor, and Supplier Sodium Saccharin Powder in Gujarat. India. Shreeji Pharma International manufacturing & gmp; supplying all products under GMP site with all types of regulatory supports.
Shreeji Pharma International is the largest manufacturer, Exporter and supplier of Sodium Saccharin Powder. Nowadays Shreeji Pharma International is one of the leading manufacturer and exporter, who is manufacturing Sodium Saccharin Powder as well as Intermediates of Sodium Saccharin Powder.
Shreeji Pharma International is exporter, supplier, distributor and manufacturers of the Active Pharmaceuticals Ingredients in many countries for many years.
Shreeji Pharma International currently exports Sodium Saccharin Powder to countries like Gulf Countries, South East Asia countries, African Countries, CIS Countries, LATAM countries, Central American Countries and in European countries.
Chemical Information
Chemical Name: Sodium 2H-1,2-benzothiazol-3-one-1,1-dioxide
Common Name: Sodium Saccharin
Molecular Formula: CHNOSNa
Molecular Weight: 205.17 g/mol
CAS Number: 128-44-9
Appearance: White crystalline powder
Solubility: Highly soluble in water
Sweetness Level: 300500 times sweeter than sucrose (table sugar)
pH (1% solution): 6.5 7.5
Description & Uses
Sodium Saccharin is a high-intensity artificial sweetener used in various industries, including food, pharmaceuticals, and personal care. It provides zero-calorie sweetness and is often combined with other sweeteners to improve taste profiles.
Applications
Food & Beverage Industry
Soft drinks, juices, and flavored water: Used as a sugar substitute.
Tabletop sweeteners: Found in sugar-free products like tablets and powders.
Baked goods & confectionery: Used in chewing gum, candy, and desserts.
Processed foods: Found in low-calorie jams, sauces, and salad dressings.
Pharmaceuticals
Used to mask bitterness in liquid medications, syrups, and chewable tablets.
Commonly included in oral care products like toothpaste and mouthwash.
Personal Care & Cosmetics
Added to lipsticks, lip glosses, and flavored cosmetics to enhance sweetness.
Used in oral hygiene products to improve taste.
Animal Feed
Used as a sweetener in livestock feed to improve palatability.
Properties & Benefits
Extremely Sweet: 300500 times sweeter than sucrose, allowing for small usage amounts.
Heat Stable: Suitable for cooking and baking applications.
Zero-Calorie & Non-Nutritive: Does not contribute to caloric intake.
Long Shelf Life: Stable in both acidic and alkaline conditions.
Safety & Precautions
Regulatory Approval: Approved by regulatory agencies such as the FDA, EFSA, and WHO, but with acceptable daily intake (ADI) limits.
Sensitivity Concerns: Some individuals may have sensitivity to saccharin, though rare.
Storage: Store in a cool, dry place, away from direct sunlight and moisture.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+










 : nilesh.sheth70
: nilesh.sheth70
